Abstract
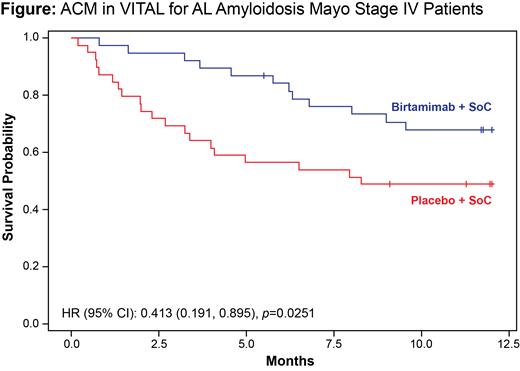
Background: Light chain (AL) amyloidosis is a rare, progressive, and typically fatal hematologic disorder caused by plasma cells that produce misfolded AL protein, resulting in deposits of amyloid in tissues and organs that cause organ dysfunction and failure. Birtamimab is an investigational monoclonal antibody designed to neutralize circulating soluble amyloid and deposited insoluble amyloid, thus promoting the phagocytic clearance of amyloid deposits. In 2018, the global phase 3 VITAL study in newly diagnosed, treatment-naïve patients was terminated based on a futility analysis of the composite primary endpoint (time to all-cause mortality [ACM] or time to cardiac hospitalization >90 days after first study drug infusion); the final hazard ratio (HR) numerically favored birtamimab + standard of care (SOC) over placebo + SOC (0.835, 95% CI 0.5799, 1.2011; p=0.330). Post hoc analysis of ACM over 9 months revealed a pronounced survival benefit (HR=0.413, 95% CI 0.191, 0.895; p=0.025; Figure) in a subgroup of patients at high risk for early mortality (Mayo stage IV). At 9 months, the proportions of surviving patients were 74% and 49% in the birtamimab + SOC and placebo + SOC groups, respectively. Post hoc analyses of secondary endpoints in this subgroup also supported clinical and functional benefits of birtamimab + SOC, with clinically meaningful improvements observed in health-related quality of life (assessed with 36-Item Short Form Health Survey version 2; SF-36v2) and 6-minute walk test (6MWT) distance (both nominal p<0.05) at 9 months. Across all birtamimab clinical trials, no drug-related deaths, dose-limiting toxicities, or major risks were identified.
Aims: To evaluate the efficacy and safety of birtamimab + SOC versus placebo + SOC in Mayo stage IV patients with AL amyloidosis by assessing time to ACM over 9 months.
Methods: The phase 3, double-blind, placebo-controlled AFFIRM-AL study will enroll up to 150 Mayo stage IV patients with newly diagnosed, untreated AL amyloidosis. Patients will receive either 24 mg/kg intravenous birtamimab or placebo every 28 days (both arms will also receive SOC, defined as concomitant chemotherapy with a first-line bortezomib-containing regimen). Patients will be randomly assigned 2:1 to birtamimab or placebo and will be stratified at randomization based on their 6MWT distance (<300 meters vs ≥300 meters). The primary efficacy endpoint of AFFIRM-AL is time to ACM using a log-rank test. Secondary endpoints are change from baseline to month 9 in SF-36v2 and 6MWT distance. Safety endpoints include adverse events, clinical laboratory observations, and immunogenicity analyses. An interim efficacy analysis is planned when ~50% of the events have occurred.
Results: Given the >50% relative risk reduction for ACM observed in the post hoc analysis of VITAL for the AL amyloidosis subpopulation of patients with Mayo stage IV disease, the phase 3 AFFIRM-AL study is designed to confirm this effect of birtamimab under a Special Protocol Assessment agreement with the US FDA.
Conclusion/Summary: Effective treatments to improve survival in AL amyloidosis are needed, particularly for patients with advanced cardiac involvement, as median overall survival for those with Mayo stage IV disease is approximately 6-9 months. Birtamimab is the only investigational therapeutic in which a survival benefit has been observed, in a post hoc subgroup analysis of VITAL in patients with AL amyloidosis with advanced cardiac involvement. AFFIRM-AL is expected to initiate in mid-2021. (NCT04973137)
Gertz: Alnylam, Amgen, Annexon, Appellis, Celgene, Ionis/Akcea, Janssen, Medscape, Physicians Education Resource, Prothena, Research to Practice: Other: personal fees; Spectrum: Other: personal fees, Research Funding; AbbVie: Other: personal fees for Data Safety Monitoring board; Teva, Johnson and Johnson, Medscape, DAVA oncology: Other: speaker fees; Pharmacyclics, Proclara: Other: Advisory Board; i3Health: Other: development of educational materials; Springer Publishing: Patents & Royalties. Tripuraneni: Prothena Biosciences Inc.: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties: related to birtamimab (NEOD001). Kinney: Prothena Biosciences Inc.: Current Employment, Current holder of stock options in a privately-held company, Patents & Royalties: related to birtamimab (NEOD001).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal